Our world is on the verge of ecological tipping, carbon neutrality has evolved from an ambitious goal to an urgent necessity. Industrial emissions especially carbon dioxide is the biggest obstacle in going green again. Modern research and experiments have enabled scientists to devise ways to have CO₂ reaction into valuable renewable fuels as compared to treating them as inevitable waste products. Among all the methods the most promising method is the catalytic-two step process that converts CO₂ into useable liquid fuels of hydrocarbons. The process uses advanced catalytic technologies, cutting-edge material science, and renewable hydrogen creating a very sustainable fueling cycle.
Understanding Industrial Emissions
The industrial sector is by far the biggest contributor to global CO₂ emissions. Petrochemical refining, cement production, steel manufacturing, power generation, and aviation manufacturing have a significant share in the atmospheric carbon buildup. The positive we can take from the industrial sector emissions is that the high concentrations provide an opportunity for direct capture and conversion, unlike emissions from domestic travel or household energy consumption.
The environmental impacts of uncontrolled industrial emissions are staggering. Rising atmospheric levels of carbon dioxide and CO₂ reactions have intensified climate change, altered ecosystems, and contributed to ocean acidification. Economically, industries are facing rising carbon taxes, reputational risks, and regulatory pressure. Hence, leading the industrial sector to invest more in carbon-emission reduction technologies.
Traditional carbon mitigation methods involve capturing and storing (CCS), aiming to sequester CO₂ underground. However, this can in the long increase volcanic eruptions and earthquakes. Additionally, these methods are cost-prohibitive and lack economic returns. On the other side, converting CO₂ into renewable fuel can convert an environmental liability into an asset.
Also Read: Europe invests in Sateliot’s Satellite Connectivity Vision
Science Behind CO₂ Conversion Reaction
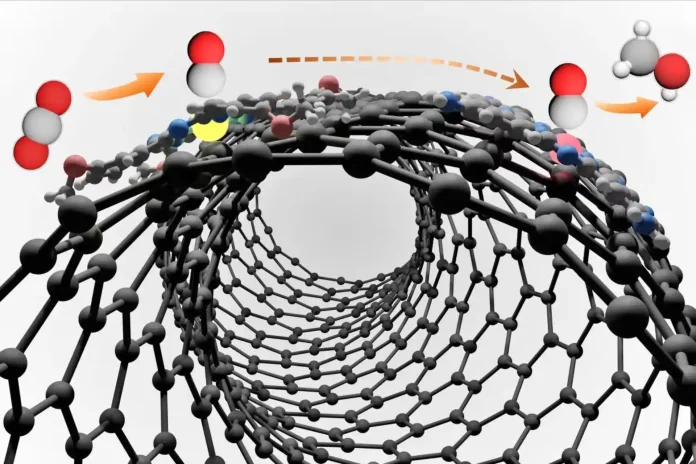
Catalytic reactions are the backbone of carbon transformation. Catalysts are chemical substances that only speed up the reaction without being consumed. Metal catalysts, especially those based on cobalt, nickel, ruthenium, and copper play’s critical role in breaking CO₂. carbon dioxide has strong molecular bonding that is difficult to break making it a very stable gas. However, the challenge in this conversion is to get efficiency while maintaining selectivity – ensuring the CO₂ reaction gives the desired results instead of unwanted byproducts.
Advancements in nanotechnology have helped scientists to design and form highly efficient catalysts with precise atomic structures, enhancing both stability and activity. The result of these advancements is the catalytic process that enables CO₂ transformation into fuel with minimal energy input.
The Two-Step Conversion Process
The reaction of transforming CO₂ into fuel follows a two-step engineered sequence.
Step 1: Electrochemical CO₂ Reduction
In the first step, CO₂ is reduced to carbon monoxide or other intermediates. The process involves the application of electric current to CO₂ in the presence of a metal catalyst. This breaks it into more reactive substances.
Step 2: Catalytic Hydrogenation
In the next step, the CO and the other CO₂ intermediates react with hydrogen (H₂) under controlled conditions to form hydrocarbons such as methanol and other liquid fuels. This process mimics the natural process of fuel formation but is catalyzed by precise catalytic engineering.
Renewable Hydrogen Availability
The possibility of CO₂ reaction to convert into fuel depends on the availability of hydrogen. Green hydrogen produced by renewable electrolysis is the most preferred input, ensuring a neutral process with no carbon footprint. Electrolysis splits water into hydrogen and oxygen using electricity. The electricity comes ideally from solar or wind energy. However, the cost of green hydrogen is acting as a barrier to its use as for two-step mechanism CO₂ reaction. Ongoing research is thriving hard to improve the efficiency of the process while reducing the cost of green hydrogen.
Potential Products and Their Applications
Some of the most promising fuel derivatives of CO₂ and their applications are listed below;
- Synthetic Hydrocarbons: they are specially engineered to replace fossil fuels in aviation, heavy-duty transportation, and maritime shipping.
- Renewable Gas Alternative: derivates of CO₂ such as methane and syngas (mix of hydrogen and carbon monoxide) integrate seamlessly into the existing energy infrastructure.
- Methanol: this is the most common industrial chemical used as a liquid fuel to prototype for chemicals or plastics.
Industrial Adoption and Challenges
For the large-scale adoption of the mechanism, industries need to retrofit existing infrastructure to accommodate carbon dioxide conversion plants. Moreover, policy support for carbon credits and subsidies can be crucial in driving investment in the cause. Additionally, regulatory frameworks must devise policies for incentivizing CO₂ utilization to ensure environmental integrity.
Conclusion
The catalytic two-step process for converting industrial CO₂ reaction into reusable fuel represents a potential paradigm shift in climate impact and energy efficiency of the carbon footprint. The integration of advanced catalysis, innovative engineering, and renewable hydrogen industries can move toward sustainable operations. However, for this vision to come to life, global collaboration is a must that requires governments, world forums, researchers, and industries to work together to make CO₂ a valuable resource instead of an environment-depleting source. The sustainable future hinges on such innovations but they are nothing without proper implementation of a workable action plan.